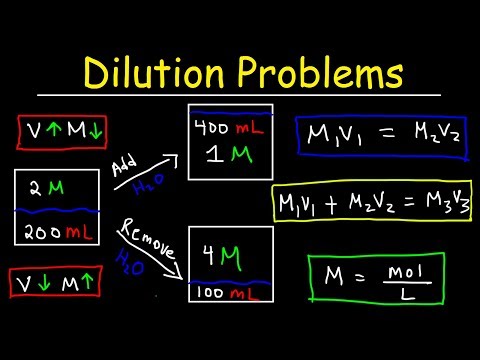
How To Find Moles Using Concentration And Volume Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution.
The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. An aqueous solution consists of at least two components, the solvent and the solute . Usually one wants to keep track of the amount of the solute dissolved in the solution. One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass.
To do this measure called molarity is commonly used. Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. For example, the known molecular weight of a chemical can be used along with the desired solution volume and solute concentration to determine the mass of chemical needed to make such a solution. To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed as molarity, the number of moles of solute in 1 L of solution.
Figure 4.6 "Preparation of a Solution of Known Concentration Using a Solid Solute" illustrates this procedure for a solution of cobalt chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution. For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure 4.7 "Preparation of 250 mL of a Solution of (NH", for some substances this effect can be significant, especially for concentrated solutions.
V is volume of solution in liters in which the indicated mass of solute must be dissolved to make the desired molar concentration . Note that V is the final or total volume of solution after the solute has been added to the solvent. To calculate molarity, divide the number of moles of solute by the volume of the solution in liters. Once you have the molar mass, multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles. Finally, divide the number of moles by the volume of the solution to get the molarity. The units of molar concentration are moles per cubic decimeter.
They are noted as mol/dm³ as well as M (pronounced "molar"). In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way.
To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark. The flasks have very good calibration so volumes are commonly known to at least four significant figures.
The definition of molarity means that you can find the molarity of a solution if you know the total number of moles of the solute and the total volume of the solution. So, in order to calculate the concentration of a solution , you need to divide moles of solute by total volume. Molality is an intensive property of solutions, and it is calculated as the moles of a solute divided by the kilograms of the solvent. Unlike molarity, which depends on the volume of the solution, molality depends only on the mass of the solvent.
Since volume is subject to variation due to temperature and pressure, molarity also varies by temperature and pressure. In some cases, using weight is an advantage because mass does not vary with ambient conditions. For example, molality is used when working with a range of temperatures.
Concentrations are often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications. Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter.
These concentrations and their units are summarized in Table 4.1 "Common Units of Concentration". We then convert the number of moles of solute to the corresponding mass of solute needed. Molar concentration is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solution.
In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol⋅dm−3 in SI unit. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M. To avoid confusion with SI prefix mega, which has the same abbreviation, small caps ᴍ or italicized M are also used in journals and textbooks.
Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute per kilogram of solvent . Molecules in a litre or even a cubic centimetre is enormous, it has become common practice to use what are called molar, rather than molecular, quantities. A mole is the gram-molecular weight of a substance and, therefore, also Avogadro's number of molecules (6.02 × 1023). Thus, the number of moles in a sample is the weight of the sample divided by the molecular weight of the substance; it is also the number of molecules in the sample divided by Avogadro's number.
Concentration in moles per litre (i.e., molarity) is usually designated by the letter M. In an ionic solution, ionic strength is proportional to the sum of the molar concentration of salts. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration . You can also calculate the mass of a substance needed to achieve a desired molarity.
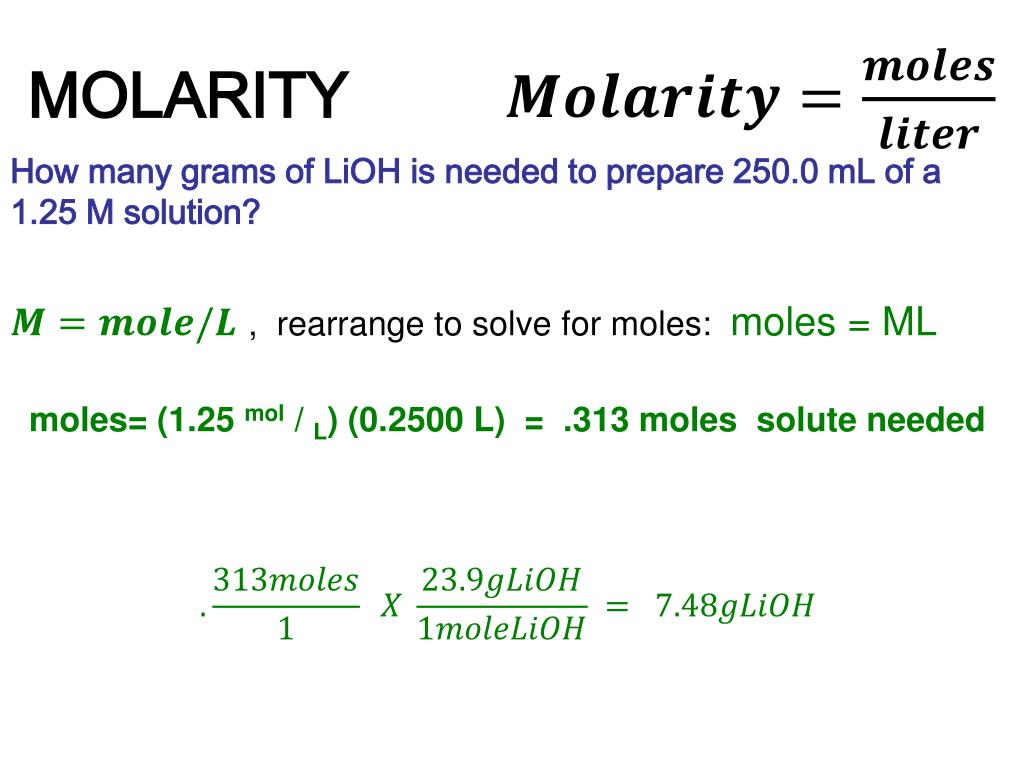
This article will provide you with the molarity definition and the molarity formula. In this example, required molarity 0.47 mol dm-3 and volume are given. When we know the molarity and volume, we can easily calculate the required amount of moles of NaOH using relationship of concentration, moles, and volume of solution. After calculating NaOH moles, required NaOH mass is calculated. The third and final step is to use the molarity formula and divide the number of moles of solute by the number liters of the solution to obtain the molarity in moles per liter.
If we take the two values from the previous step, we see that the ammonia solution is 2.9 M. This means that every liter of this solution contains 2.9 moles of ammonia. The volume in liters of a solution is equal to the mass in grams divided by the molar mass of the substance in g/mol, divided by the molar concentration in molars.
The mass in grams of a solution is equal to the molar concentration in molars times the volume in liters times the molar mass of the substance in g/mol. The molarity is just the mass in grams divided by the molar mass in g/mol divided by the volume in liters. This formula can be simplified into the following standard equation, which is most commonly referenced when calculating molarity.
The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume. To dilute a solution of known molarity, please use the Solution Dilution Calculator. To dilute a solution of concentrated acid or base of known w/w% strength, please use the Acid & Base Molarity Calculator.
So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration . Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!). Solutions are homogenous mixtures made of a solute and a solvent.
Examples of solutions include salt water, apple juice or Gatorade. One of the most important properties of a solution is its concentration, or the ratio of solute to solvent. Most of the time, the concentration of a solution is expressed using molarity, which is represented by a capital M. An online molarity calculator helps you to calculate molarity, volume, concentration and mass of a concentrated or dilute solution. You can get required answers in many units for a specific term by using this tool.
The molar concentration unit [mol/ L ] is a conventionally widely used as concentration method. It is the number of moles of target substance dissolved in 1 liter of solution. Solution concentrations are typically expressed as molarity and can be prepared by dissolving a known mass of solute in a solvent or diluting a stock solution.
Molarity is a unit of concentration, measuring the number of moles of a solute per liter of solution. The strategy for solving molarity problems is fairly simple. This outlines a straightforward method to calculate the molarity of a solution. The amount of substance in moles can be found using the molar mass of the formula and the mass of substance. Use our molar mass calculator if you need to calculate the molecular weight of the solute. Molarity is the ratio of the amount of solute in moles to the volume of solution in liters.
Molarity differs from molality in that it is a ratio of the amount of substance to volume, while molality is a ratio of the amount of substance to mass. To calculate molarity, you can start with moles and volume, mass and volume, or moles and milliliters. Plugging these variables into the basic formula for calculating molarity will give you the correct answer.
To find the concentration of this new solution you need to convert from grams to moles. This requires the use of the molar mass (given in grams/mole) of the NaCl. The molar mass of a substance is found by adding together the molar mass of the individual components. For NaCl, the two components are sodium and chloride.
Chemists use many different units for describing concentration. However, the term molarity, also known as molar concentration, is the most common way of expressing the concentration. When the reactants are expressed in mole units, it allows them to be written with integers in chemical reactions. First, let's take a closer look at what is the mole, so we can move on later to find what is molarity. Dissolved amount and totoal volume of the solution are required to calculate the concentration according to the molarity equation.
In this example, total solution volume is given as a data. Therefore we have to find the dissolved NaCl amount. Stoichiometry is the study of relative quantities in substances that undergo physical or chemical changes. Learn about stoichiometry of gases, molar volume, solutions, and how to use the four-step process to calculate relative quantities in a gas or solution. In electrolyte solutions it is common to distinguish between the solvent and the dissolved substance, or solute, which dissociates into ions.
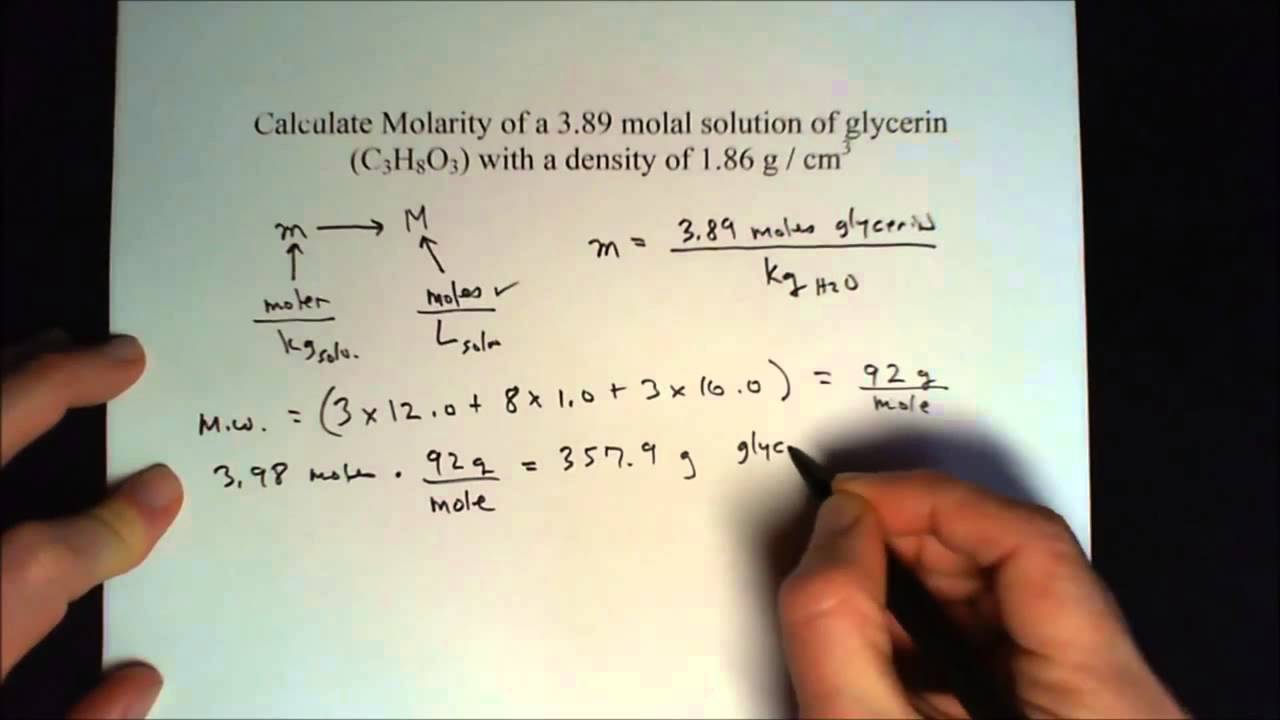
For these solutions it is useful to express composition in terms of molality, designated as m, a unit proportional to the number of undissociated solute molecules per 1,000 grams of solvent. The number of molecules or ions in 1,000 grams of solvent usually is very large, so molality is defined as the number of moles per 1,000 grams of solvent. An alternative way to define the concentration of a solution is molality, abbreviated m. Molality is defined as the number of moles of solute in 1 kg of solvent.
Would you expect a 1 M solution of sucrose to be more or less concentrated than a 1 m solution of sucrose? Diluting a stock solution is preferred because the alternative method, weighing out tiny amounts of solute, is difficult to carry out with a high degree of accuracy. Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions, such as strong acids. In this explainer, we will learn how to calculate the molar concentration of a solution from the solvent volume and mass or moles of the dissolved solute. You can get your moles by taking the molar mass of each of the elements in the solute and adding them together. Do it; the answer is in moles because the grams cancelled out.Then, go ahead and do your formula.
Molarity is not the same as concentration, although they are very similar. Concentration is a measure of how many moles of a substance are dissolved in an amount of liquid, and can have any volume units. Molarity is a type of concentration, specifically moles per liter of solution. Where mass is the mass of solute in grams, and volume is the total volume of solution in liters.
Molarity indicates the number of moles of solute per liter of solution (moles/Liter) and is one of the most common units used to measure the concentration of a solution. From mol dm-3 we know, we have to calculate the molarity. This means how many moles of solvent are dissolved in total of 1 dm3 volume of solution. The number of moles of the solute dissolved per unit volume of the solution is the definition of molarity.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.